From ivWatch

Company Strengthens International Expansion and Broadens the Availability of Continuous IV Monitoring Solutions to Strategic Markets
Newport News, VA, August 10, 2022 – ivWatch, LLC, an innovative IV safety company working to eliminate the harm caused by infiltration and extravasation events, announced three new strategic distribution partnerships internationally. Bameco in Austria, Warba Medical Supplies in Kuwait, and Mustafa Sultan Science & Industry Co, LLC in Oman have each signed exclusive agreements to promote, sell, and support ivWatch’s continuous monitoring products in their markets. ivWatch’s novel technologies will complement and bolster their existing portfolios of intravenous products provided to hospitals and other health care facilities.
ivWatch solutions continue to be available in more countries throughout the globe and with the addition of Austria, Kuwait, and Oman, clinicians will now be able to improve their bedside decision-making with real-time IV infiltration data. Continuous IV site monitoring provides accurate and precise insights to detect infiltrations and extravasations at their earliest stages. ivWatch’s proprietary algorithm detects infiltrations promptly and notifies clinicians of adverse IV therapy events often hours before they are detectable by visual or tactile examination.1
“At ivWatch, we are on a steadfast mission to improve IV safety globally and we are extremely passionate about partnering with like-minded innovators who share that same relentless pursuit to advance patient safety,” said Chuck Egress, Vice President of Business Development and Emerging Markets at ivWatch. “IV infiltrations and extravasations are a major global patient safety concern, and these new partnerships provide increased access to advanced safety solutions to help end complications and improve patient outcomes in IV therapy.”
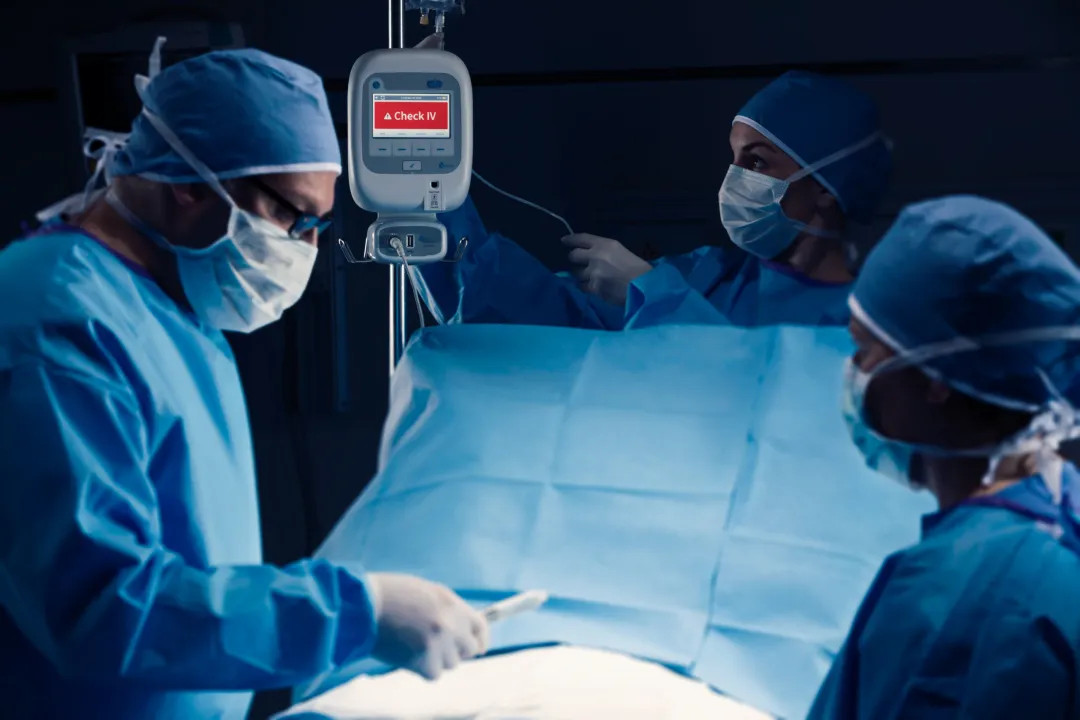
ivWatch has over 60 patents worldwide and received its first FDA clearance in 2015 for its IV site monitoring system that consists of an ivWatch Patient Monitor and a reusable optical biosensor that uses visible and near-infrared light to continuously measure changes in the optical properties of the tissue near an IV insertion site. In 2020, the company expanded its portfolio of sensor solutions and received FDA clearance for a single-use, miniaturized biosensor that is cleared for all patient age groups, sizes, and skin tones. Both sensors work seamlessly with the intuitive user interface on the Patient Monitor, which issues audible and visual “Check IV” alerts for the clinician to assess the IV site. ivWatch technology performs with high accuracy, intelligence, and low false alarm rates, which is critical to improving patient outcomes.
To learn more about ivWatch, visit www.ivWatch.com or follow the Company’s social media platforms for the latest updates on new continuous monitoring innovations in IV safety.
About ivWatch, LLC
ivWatch, LLC is a biosensor technology company focused on improving patient safety and the effectiveness of intravenous therapy. Our dedicated and passionate team is pioneering the use of optical sensors to detect adverse IV events early to minimize the risk of injury caused by infiltrations and extravasations. Using this technology, clinicians can leverage continuous monitoring to help identify infiltrations as early as possible. Our innovative IV site monitoring solutions are backed by decades of clinical research and device development. To learn more, follow us on Twitter @ivWatch, Facebook @ivWatchLLC, and LinkedIn @ivWatch-LLC, or visit www.ivWatch.com.
MEDIA CONTACT:
Erin Wendell
erin.wendell@ivwatch.com
Mobile (757) 633-4688






